2024 Guideline for the Management of Hypertrophic Cardiomyopathy
Published: mayo 08, 2024
- Reaffirmed: This 2024 AHA/ACC/AMSSM/HRS/PACES/SCMR Guideline has been reviewed and determined to be current as of May 2025. See the 2025 AHA/ACC Methodology and Policies Manual (PDF) for information on reaffirmation.
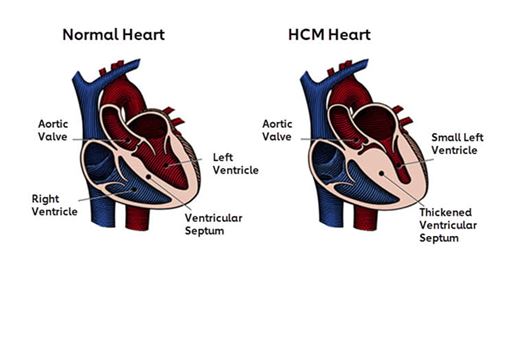
- Hypertrophic Cardiomyopathy (HCM) remains a common genetic heart disease in populations throughout the world.
- The prevalence of unexplained asymptomatic hypertrophy in young adults in the United States has been reported at about 1:500. Symptomatic hypertrophy based on medical claims data has been estimated at <1:3000 adults in the United States.
Video: 2024 Guideline for the Management of Hypertrophic Cardiomyopathy
Writing Committee Chair Steve R. Ommen, MD, FACC, FAHA and Vice Chair Carolyn Ho, MD, FAHA discuss the 2024 updates to the Hypertrophic Cardiomyopathy (HCM) Guidelines. Following FDA approval of the first cardiac myosin inhibitor mavacamten for symptomatic obstructive HCM, the guideline now includes it as an option before more invasive therapies when first-line treatments like beta blockers or calcium channel blockers are not effective. Additionally, changes in recommendations regarding exercise and activity levels are discussed, emphasizing a more inclusive approach towards physical activity for patients with HCM.
Slide Sets
- AHA Clinical Update: 2024 AHA/ACC/AMSSM/HRS/PACES/SCMR Guideline for the Management of Hypertrophic Cardiomyopathy Slide Set

Download AHA Clinical Update Slide Set (PPTX) - 2024 AHA/ACC/AMSSM/HRS/PACES/SCMR Guideline for HCM Slide Set

Download 2024 AHA Guideline Slide Set (PDF)
Related Resources
- Hub - 2024 Guideline for the Management of Hypertrophic Cardiomyopathy
- Key Patient Messages Hypertrophic Cardiomyopathy 2024 Clinical Practice Guideline
- HCM Guideline Pocket Guide
- HCM-AF Risk Calculator
- 2020 AHA/ACC Guideline for the Diagnosis and Treatment of Patients With Hypertrophic Cardiomyopathy: Executive Summary